Giant cell tumor of the maxillary bone VS central giant cell granuloma: Case report
- Corresponding Author:
- Charfeddine Abir, University Clinic of Dental Medicine, Oral Surgery Department 5000, Monastir, Tunisia
Phone: +216 25821827
E-mail: Charfeddine.abir@yahoo.fr
Abstract
Giant cell tumors are osteolytic neoplasms. They are rare in the region of the head and neck, where they are localized preferentially at the sphenoid or temporal bones, and they remain exceptional at the jaw bones. Women are more affected than men, with an age range of 25 years to 40 years. These tumors show benign histological features. However, they are locally aggressive, with a rate of malignant transformation of 10%-20% of cases. The principal differential diagnosis arises mainly with the central granuloma with giant cells. Several therapeutic modalities have been mentioned in the literature, but surgery remains the treatment of choice for this type of lesion. in the literature, there is a big confusion between giant cell tumor and central giant cell granuloma; in some articles, the authors consider them as the same pathological entity, hence the aim of our work to clarify the differences between giant cell tumor and central giant cell granuloma clinically, radiologically, histologically and cytologically by presenting a case of giant cell tumor, of rare localization in the jawbone, in a young patient aged 17, who consults with a lesion in the right maxilla and severe dental mobility.
Keywords
Giant cell tumors; Granuloma; Giant cell; Histology; Comparative; Maxilla; Surgery; Oral
Introduction
Giant Cell Tumors (GCT) was first described in 1953 as a reactive lesion to intraosseous hemorrhage following repetitive trauma [1]. These are osteolytic neoplasms with a recurrence potential of around 25% of cases, while 2% may develop pulmonary metastases [2]. The differential diagnosis is mainly with brown tumor and central giant cell granuloma, which is an uncommon, benign but aggressive osteolytic neoplasm of the craniomaxillofacial region, characterized histologically by an abundance of multinucleated giant cells uniformly distributed in a sea of spindle-shaped mesenchymal stromal cells, dispersed in fibro vascular connective tissue stroma containing areas of hemorrhage, the importance of distinguishing between these two pathologies comes from the fact that giant cell tumors have a tendency to progression, with a high recurrence rate and risk of malignant transformation [3]. In this context we will present a rare case report of a 17-year-old female patient with right maxillary TCG, and her management.
Case Presentation
A 17-year-old female patient with a history of auricular septal defect operated on 2003 and no other medical history, consulted the Oral Medicine and Surgery Department of the Monastir Dental Clinic with a right-sided latero-nasal swelling that had been evolving for 2 months. The patient mentioned the progressive appearance of tooth mobility affecting the entire right maxillary sector, from the central incisor to the second molar. The swelling was not associated with any signs of altered general condition (neurological or vascular).
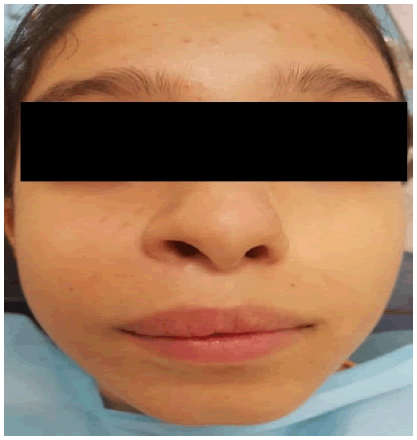
Exo-buccal examination revealed, facial asymmetry caused by -Facial asymmetry due to diffuse swelling on the right side, resulting in obliteration of the nasolabial fold. The swelling was covered by taut skin, with no change in color or rise in temperature. Examination of the cervical lymph nodes revealed no adenopathy (Figure 1).
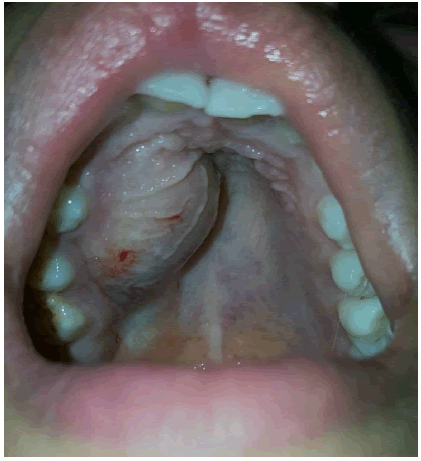
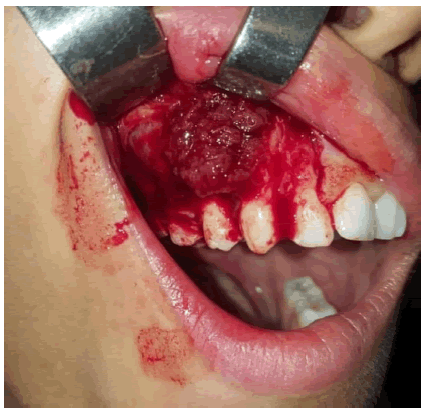
Endo-buccal examination showed, adequate oral hygiene, with tartar deposits on the vestibular and occlusal surfaces of the right maxillary first and second molars, a sign of homolateral masticatory hypofunction due to tooth mobility. The dental formula is complete, with severe grade 3 mobility of 12, 13, 14 and 15 and grade 1 mobility of 11, 16 and 17. Swelling of the maxillary right vestibular alveolar table extending from the maxillary central incisor to the second molar on the right side. The swelling is covered by a fissured, irregular mucosa with local erosions.
Inflammation of the papillae, with an irregular appearance in the free gingiva of the mobile teeth (Figure 2a and 2b).
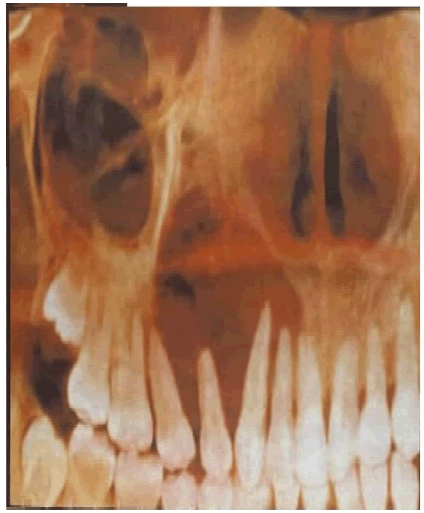
The panoramic X-ray showed a clear, well-limited image in the right maxilla extending from 11 to 15, extending to the maxilla malar junction, but without extending into the maxillary sinus or homolateral nasal cavity (Figure 3).
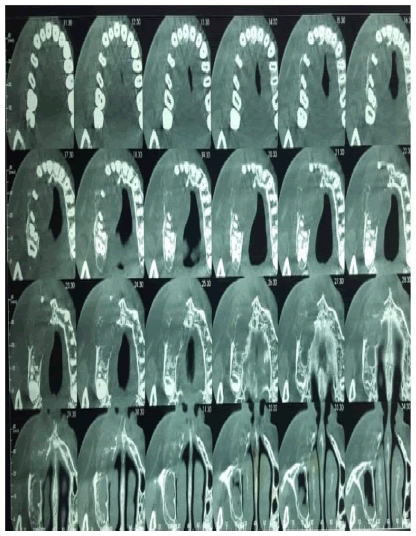
The Cone beam showed a hypodense, osteolytic image of the right maxilla, with a pyramidal shape with a lower base from 11 to 15, of polycyclic contour, with local disruption of the vestibular and palatal cortical. The sinus on this side showed signs of chronic sinusitis with thickening of the sinus wall (Figure 4).
According to the clinical and radiological data, the diagnosis of giant cell tumor, central giant cell granuloma and brown tumor were among the probable diagnoses, without ruling out the risk of a malignant lesion. Biological examinations showed: serum calcium: 2.35 mmol/L (2.25 mmol/L-2.6 mmol/L), serum phosphorus: 1.12 mmol/L (0.8 mmol/L-1.4mmol/L), parathyroid hormone: PTH=36 PG/ML (15 PG/ML-65 PG/ML). These values are within physiological norms, ruling out the diagnosis of maxillary brown tumor.
A biopsy was performed in the Oral Medicine and Surgery Department of the Monastir Clinic, in orange sections, straddling the gap between healthy and pathological tissue, and involving both superficial and deep tissue (Figure 5).
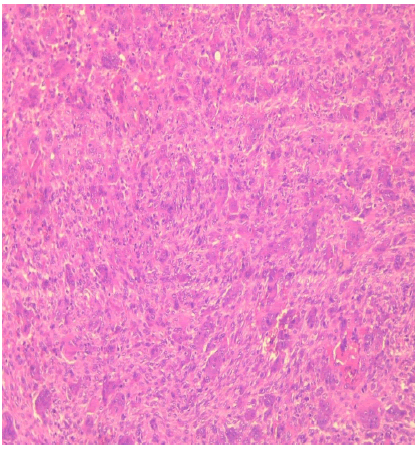
The pathology report showed a benign tumor proliferation consisting of monomorphic ovoid or spindle-shaped cells with poorly defined cytoplasm and nuclei with fine, nonatypical chromatin. These cells are interspersed with numerous multi-nucleated giant cells of variable size, harmoniously distributed. The base contains a few fragments of bone wedged between the tumor cells. All these data do not suggest cytonuclear atypia, thus ruling out signs of malignancy. In conclusion, it was a giant cell tumor of the right maxillary bone (Figure 6).
The patient was treated at the maxillofacial surgery department of CHU Sahloul in Sousse, by surgery with excision of the entire lesion. Anatomopathological examination of the entire surgical specimen after removal of the lesion showed that it was within healthy limits, and that the entire lesion had been removed.
The patient benefited from a maxillofacial prosthesis, performed rapidly after surgery in view of the psychological consequences of surgery, replacing the extracted teeth, as well as the loss of tissue in the maxilla after surgery.
Discussion
Giant cell tumors of bone are rare primary tumors that occur mainly in the extremities of long bones. It is rarely discovered in the bones of the face and skull, where it preferentially involves the sphenoid and temporal bones.
Maxillary involvement, as described in our case, is exceptional. This tumor presents benign characteristics; however, it can be locally aggressive and may even metastasize to the lungs [3].
According to the literature, no family history has been associated with TCG [2,4]. This lesion occurs more frequently in women between the ages of 25 years and 40 years. Our case is a 17-year-old girl, which makes it unusual [2,4-7].
Clinically, Franco Bertoni et al describe TCG as a reddish-brown, vascular mass, sometimes dark, with a soft, granular, fleshy consistency [3]. The reddish appearance is explained by the presence of hemorrhagic areas, characteristic of these lesions.
N. Gholizadeh et al, describe TCG as a tumefaction giving a noticeable facial asymmetry, often asymptomatic and slow-growing, this lesion can appear aggressive and painful in around 25% of cases [4]. In our patient's case, the lesion was fast-growing but painless.
This type of lesion develops without neurological manifestations, and the associated teeth may, at an early phase, show various degrees of mobility, but retain their vitality. In our case, the absence of neurological signs and the presence of degree 3 tooth mobility are similar to those reported in the literature.
According to Saw S et al, Bertoni F et al, N. Gholizadeh et al and Gupta M, there are no radiological pathognomonic signs of TCG, but it most often presents as a radio-clear, poorly limited, uni or multilocular lesion, showing variable expansion and cortical destruction while respecting adjacent structures [2-5].
Biological tests were performed in all articles presenting osteolytic lesions that suggested the diagnosis of brown tumor. These analyses included serum calcium, phosphate and parathyroid hormone levels. Increased values are suggestive of brown tumors, whereas normal values rule out this diagnosis. For our patient, these values are within physiological values.
Comparing the histological appearance described in our patient's pathological examination report with the histological appearance of TCG in the literature, several points of similarity are noted. Indeed, according to S. Saw et al, N. Gholizadeh et al, I. Barthélémy et al and P. L. Auclair et al, giant cells are only osteoclasts in TCG, resulting from the fusion of recruited circulating monocytes, they are numerous, and almost uniformly distributed in a matrix poor in collagen and rich in spindle cells [2,4,6,8]. Mitosis is accentuated but not abnormal.
This tumor must be distinguished from central giant cell granuloma. Indeed, these two lesions are sometimes considered to be the same pathological process, the isoclinic expression of which is modified by age and skeletal topography [6].
Central Giant Cell Granuloma (CGCG) was first defined by Jaffe in 1953. It has been described as a benign process confined to the mandible or maxilla and may be related to trauma and hemorrhage, infections, or developmental abnormalities. It often appears in the dentate alveolar region and can be seen at any age, developing with predilection in the mandible (twice as frequently as in the maxilla) [2].
Clinically, TCG resembles the aggressive type of GCCG. The aggressive form is more explosive and rapidly progressive. The lesion is large (over 5 cm). It is associated with pain, mucosal ulceration, sensitivity disturbances, bone cortical crossing and root resorption [6].
Histologically, TCG differs from GCCG by the presence, within the granuloma, of fibroblast-like spindle cells and multi-nucleated foreign-bodylike giant cells distributed irregularly around a dense interstitial capillary network, hemorrhagic foci with hemosiderin deposits, at the periphery of the granuloma, trabeculae of osteoid or bony tissue can be observed [6].
The stroma in giant cell granuloma is collagenized or edematous, whereas in giant cell tumor, the stroma is composed of plump tumor cells, which are mesenchymal in origin and ultrastructurally share features with fibroblasts or osteoblasts [2- 5].
Cytologically, TCG giant cells have more nuclei than CGCG giant cells, and these nuclei are more tightly clustered and larger in size [2-4,6,9,10].
Expression of the P63 protein, found only in TCGs, can be a diagnostic element of certainty [2].
TCG has a higher recurrence rate, with the risk of metastasis and malignant transformation (in 10%-20% of cases transform into sarcomas), hence the importance of distinguishing between TCG and GCRG [4-6].
Optimal management of TCG and CGCG includes surgical resection, sometimes supplemented by postoperative radiotherapy if primary excision is considered incomplete. Surgical reconstruction may be considered if a large primary excision was necessary. Conservative treatment based on curettage alone has been associated with high recurrence rates. Combination chemotherapy with methotrexate, adriamycin and cyclophosphamide is usually reserved for cases that remain inadequately controlled by surgery [2].
The following table summarizes the clinical, radiological, histological and cytological comparisons between giant cell tumors and central giant cell granulomas.
Conclusion
Giant cell tumors rarely occur in the head and neck region, preferentially localizing to the sphenoid and temporal bone. The differential diagnosis is essentially with giant cell granuloma, where they are confused by their clinical and radiological appearance. Histological examination is the key to differentiating between these two pathological entities.
Declaration
â Informed consent
Written informed consent was obtained from the parents of the patient.
â Conflict of interest
The authors declare no conflict of interest
â Authors’ contributions
Abir Charfeddine: wrote the article.
Chokri Abdellatif: involved in the writing and editing of the article.
Dammak Nouha, Zouaghi Hela: followed up the patient and involved in the writing of the article.
Jamil Selmi: reviewed the article for important intellectual content.
References
- Alireza Karimi, Y., Sazgar, A. A., Kouhi, A. Multicentric giant cell tumor: metachronous central and peripheral involvement. Ear Nose Throat J. 91(1), 37-39 (2012).
- .Saw, S., Thomas, N., Gleeson, M. J., et al. Giant cell tumour and central giant cell reparative granuloma of the skull: do these represent ends of a spectrum? A case report and literature review. Pathol Oncol Res. 15, 291-295 (2009).[ Google Scholar]
[CrossRef]
- Bertoni, F., Unni, K. K., Beabout, J. et al. Giant cell tumor of the skull. Cancer. 70(5), 1124-1132 (1992).
- Gholizadeh, N., Mehdipour, M., Bahramian, A. et al. Central Giant Cell Granuloma of the Posterior Maxilla: A Case Report. Oral Surg Oral Med Oral Pathol Oral Radiol. 119(3), e218 (2015).
- Gupta, M., Gupta, M., Singh, S., et al. Central giant cell granuloma of the maxilla. Case Rep. 2013, (2013).[ Google Scholar]
[CrossRef]
- Barthélémy, I., & Mondié, J. M. Tumeurs et pseudotumeurs des maxillaires riches en cellules géantes. Rev Stomatol Chir Maxillo-fac. 110(4), 209-213 (2009).
- Bernard, F., Troude, L., Bouvier, C., et al. Giant cell reparative tumor: An exceptional differential diagnosis for a lytic lesion of the temporal bone. Neurochirurgie. 62(6), 332-335 (2016).
- Auclair, P. L., Cuenin, P., Kratochvil, F. et al. A clinical and histomorphologic comparison of the central giant cell granuloma and the giant cell tumor. Oral Surg Oral Med Oral Pathol. 66(2), 197-208 (1988).
- de Lange, J., van den Akker, H. P., & van den Berg, H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 104(5), 603-615 (2007).[ Google Scholar]
[CrossRef]
- Wang, C., Song, Y., Peng, B., et al. Expression of c-Src and comparison of cytologic features in cherubism, central giant cell granuloma and giant cell tumors. Oncol Rep. 15(3), 589-594 (2006).